New research may explain severe virus attacks on the lungs
In some cases, immune cells in the lungs can contribute to worsening a virus attack. In a new study, researchers at Karolinska Institutet describe how different kinds of immune cells, called macrophages, develop in the lungs and which of them may be behind severe lung diseases. The study, which was published in Immunity, may contribute to future treatments for COVID-19, among other diseases.

The structure of the lungs exposes them to viruses and bacteria from both the air and the blood. Macrophages are immune cells that, among other things, protect the lungs from such attacks. But under certain conditions, lung macrophages can also contribute to severe lung diseases, such as chronic obstructive pulmonary disease (COPD) and COVID-19. To date, research on the development of human lung macrophages has been limited.
Macrophages can have different origins and develop, among other things, from white blood cells, monocytes, that are divided into different genetically determined main types. In humans, two of these are “classical” CD14+ monocytes and “non-classical” CD16+ monocytes.
Single-cell RNA analysis of monocytes and macrophages
In a new study at Karolinska Institutet, researchers have used a model to study the development of lung macrophages directly in a living lung. This has been combined with a method to study gene activity in individual cells, RNA sequencing, and thereby discovered how blood monocytes become human lung macrophages.
“In our study, we show that classical monocytes migrate into airways and lung tissue and are converted into macrophages that protect the health and function of the lungs. We have also identified a special kind of monocyte, HLA-DRhi, which is an intermediate immune cell between a blood monocyte and an airway macrophage. These HLA-DRhi monocytes can leave the blood circulation and migrate into the lung tissue,” says Tim Willinger, Associate Professor at the Department of Medicine, Huddinge, Karolinska Institutet, who led the study.
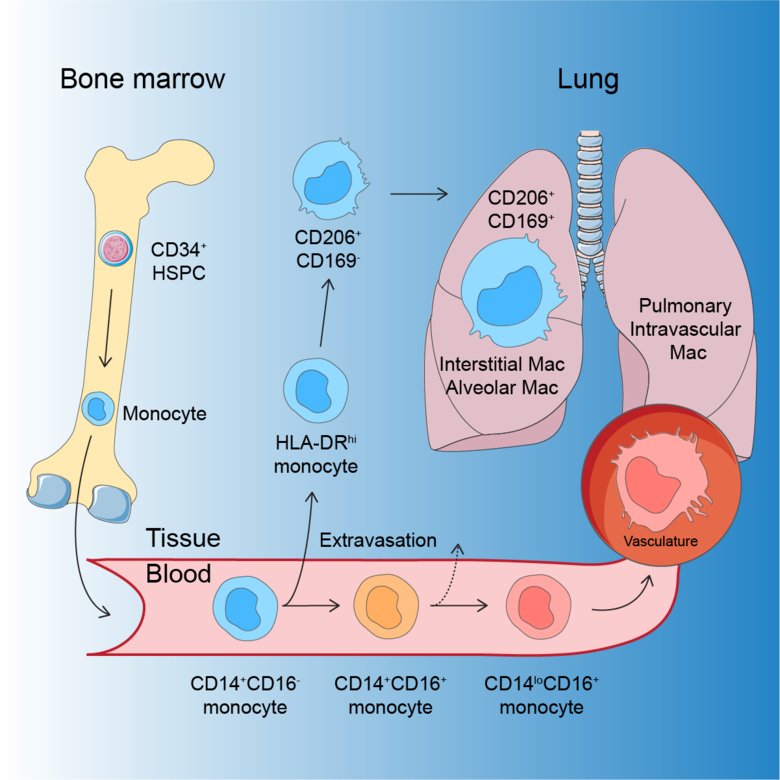
The non-classical monocytes, however, develop into macrophages in the many blood vessels of the lungs and do not migrate into the lung tissue.
Probable connection to lung disease
“Certain macrophages in the lungs probably have a connection to a number of severe lung diseases. In respiratory infections, for example, monocytes in the lungs develop into macrophages, which combat viruses and bacteria. But a certain type of macrophage may also contribute to severe inflammation and infections,” says the study’s first author Elza Evren, a doctoral student in Tim Willinger’s research team.
In an infection with the novel coronavirus, SARS-COV-2, which causes COVID-19, researchers believe that protective, anti-inflammatory macrophages are replaced by pro-inflammatory lung macrophages from blood monocytes.
“The existence of these blood monocyte-derived macrophages has been shown in other studies to correlate with how severely ill a person becomes in COVID-19 and how extensive the damage to the lungs is. Patients with severe COVID-19 also have fewer HLA-DRhi monocytes in their blood, probably because they move away from the blood into the lungs. Given their important role in rapid inflammatory responses, our results indicate that future treatments should focus on inflammatory macrophages and monocytes to reduce lung damage and mortality from severe COVID-19,” says Tim Willinger.
The research is financed by the Swedish Research Council, Karolinska Institutet, Centre for Innovative Medicine (CIMED)/Region Stockholm, the Swedish Heart-Lung Foundation, and the Swedish Cancer Foundation. There are no reported conflicts of interest.
Publication
“Distinct developmental pathways from blood monocytes generate human lung macrophage diversity”. Elza Evren, Emma Ringqvist, Kumar Parijat Tripathi, Natalie Sleiers, Inés Có Rives, Arlisa Alisjahbana, Yu Gao, Dhifaf Sarhan, Tor Halle, Chiara Sorini, Rico Lepzien, Nicole Marquardt, Jakob Michaëlsson, Anna Smed-Sörensen, Johan Botling, Mikael C. I. Karlsson, Eduardo J. Villablanca, Tim Willinger. Immunity, online 30 December 2020, doi: 10.1016/j.immuni.2020.12.003.
