Molecular patterns behind unstable atherosclerosis identified
A large-scale multi-omics study from Karolinska Institutet, published in Genome Medicine, provides new insight into the molecular mechanisms underlying unstable atherosclerosis, one of the most important causes of ischemic stroke and myocardial infarction.
The Vascular Surgery research group under the leadership of Associate Professor Ljubica Matic and Professor Ulf Hedin at the Department of Molecular Medicine and Surgery, has published a large-scale multi-omics study that sheds new light on the molecular mechanisms underlying unstable atherosclerosis, a major cause of ischemic stroke and myocardial infarction. The study has been performed together with researchers at Novo Nordisk and is the result of a collaborative strategic partnership between Karolinska Institutet and the company since 2019. The study is also part of a large EU Horizon Tools project NextGen, an international consortium of 20 partners set to advance AI and multi-modal data integration in personalised cardiovascular medicine.
Extensive patient cohort and biobank-based analysis
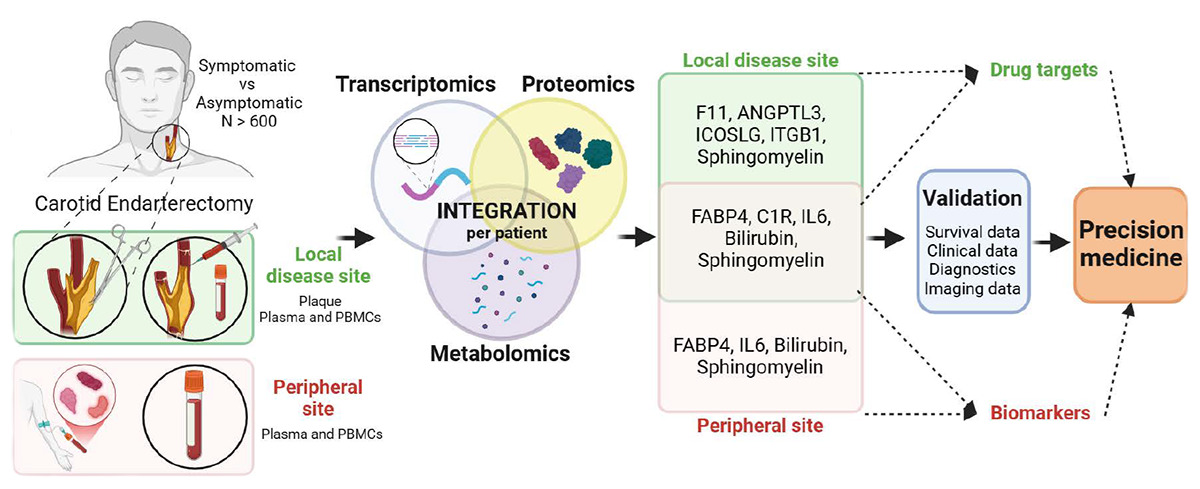
Using samples from the Biobank of Karolinska Endarterectomies (BiKE), the team analysed matched atherosclerotic plaques, circulating immune cells and blood plasma from more than 700 patients undergoing carotid endarterectomy. Patients with symptomatic (unstable) disease were compared with those with asymptomatic (stable) disease to identify molecular patterns associated with plaque instability.
By integrating transcriptomic, proteomic and metabolomic data per patient with a supervised machine-learning method, the researchers identified coordinated molecular signatures that distinguished symptomatic from asymptomatic patients across both the local disease site and peripheral circulation. The analyses confirm the importance of inflammation, coagulation, necroptosis and lipid metabolism in unstable disease, while also highlighting less explored pathways, including sphingomyelin metabolism and bilirubin-related processes.
Several molecules consistently emerged as key markers, including IL6, FABP4, ANGPTL3, ICOSLG, F11, and multiple sphingomyelins. Some of these markers were associated with commonly used cardiovascular medications, while others - most notably ANGPTL3 - were not influenced by current therapies and are already under clinical trials. Importantly, identified molecular signals were also linked to long-term risk of major cardiovascular and cerebrovascular events after surgery.

“By combining deep, layered molecular profiling with clinical data and long-term follow-up, the study provides a systems-level view of atherosclerotic instability in humans. The findings support the potential for improved patient stratification, as well as the identification of novel biomarkers and therapeutic targets, advancing efforts toward precision medicine in cardiovascular disease,” says Ljubica Matic, Associate Professor and one of the two leaders of the study.
The strategic partnership with Novo Nordisk has been instrumental for expanding datasets in the biobank, providing access to expertise and company resources. For the research group, the collaboration has thereby elevated the potential of research in human atherosclerosis and even if the partnership is formally finalised, more studies are under way.
Visual summary of the study
The study was performed as part of the strategic research partnership between Karolinska Institutet and Novo Nordisk, Denmark. The work was also funded by a research grant from the European Union’s HORIZON-HLTH-2023-TOOL-05 program with grant agreement NextGen, the Swedish Research Council, Swedish Heart-Lung Foundation and Karolinska Institutet Consolidator program.
Publication
"Multi-omics data integration from patients with carotid stenosis illuminates key molecular signatures of atherosclerotic instability", Das V, Narayanan S, Zhang X, Bergman O, Djordjevic D, Kronqvist M, Chemaly M, Karadimou G, Sundman S, Prasad I, Buckler AJ, Knape KC, Michaelsen NB, Hedin U, Matic L. Genome Medicine, online 6 February 2026, doi: 10.1186/s13073-026-01601.
