KI researchers to evaluate effect of hyperbaric oxygen treatment against COVID-19
Researchers at Karolinska Institutet are coordinating a recently initiated multi-national clinical trial that will evaluate the effect of hyperbaric oxygen treatment (HBO) in severe cases of COVID-19. A total of 200 adults admitted to hospital with moderately severe COVID-19 pneumonia will be recruited. The trial could start as early as next week at a hospital in Region Blekinge, Sweden.

Anders Kjellberg, PhD-student at the Department of Physiology and Pharmacology (FyFa) at Karolinska Institutet and physician at the Karolinska University Hospital, coordinates the trial and tells us more about the project.
Tell us about the purpose of the clinical trial.
The purpose is to evaluate the safety and efficacy of hyperbaric oxygen (HBO) treatment in COVID-19 disease with severe pneumonia. Inflammation out of control is a significant feature of the severe cases of COVID-19 and there is currently no effective cure. HBO treatment is used daily all over the world to treat certain types of inflammation and infections and we think it could have a suppressive effect on inflammation in more severe cases of COVID-19. We hope that the treatment will reduce the need for intensive care admission, the risk of multi-organ failure and mortality in COVID-19 patients.
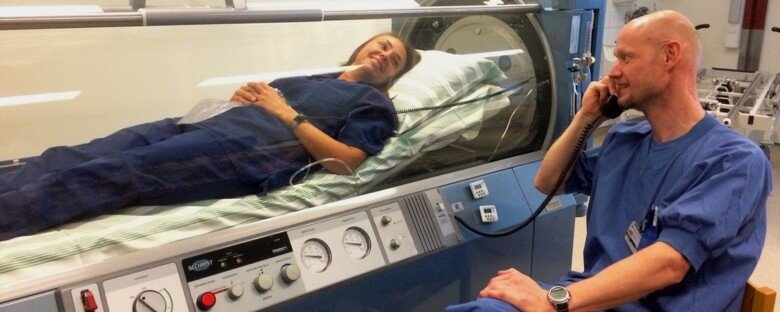
What is hyperbaric oxygen (HBO) treatment?
HBO treatment involves breathing 100 percent oxygen in a pressurized chamber. The method has been used for almost 100 years, partly to treat divers with decompression sickness, and the risks are considered low. The underlying mechanisms are not completely understood, but we know that HBO can suppress the part of the immune response that is out of control in some patients with COVID-19 and makes them very ill. Fortunately, HBO does not seem to negatively affect the part of the immune response that is needed to kill the virus. Therefore, we think that a few short, intense treatments with HBO could reduce the overall need for oxygen and keep the patient from deteriorating to the point where they need mechanical ventilation in the ICU.
What does it mean for the trial participants?
We will ask a total of 200 adults admitted to hospital, mainly in Europe, with moderately severe COVID-19 if they want to participate in the trial. After informed consent and controls to ensure all requirements for participation in the trial are met, half of the participants will be randomized to either the HBO group or to the group that will receive care according to the best practice at the time. Both groups will receive standard treatments for COVID-19. The participants will receive a maximum of five HBO treatments in the first seven days. In each treatment, the subjects will breath oxygen in a pressurized chamber for 30 to 60 minutes. During this time, the ambient pressure will increase to a level corresponding to a dive of 14 meters under water. We will collect several blood samples from the participants to study the immune response, signs of organ failure and evaluate a number of safety and efficacy variables.
When will you know if it works?
The trial will last for 30 days. The first safety evaluation will be done when 20 subjects have completed the trial and within months we will know if there is a positive effect.
The project is supported by grants from the Swedish Research Council made available through co-researcher Kenny Rodriguez-Wallberg, associate professor and co-investigator at the Department of Oncology-Pathology. Peter Lindholm, associate professor at FyFa is senior researcher and sponsor representative.
More information about the clinical trial is available at clinicaltrials.gov.
