New method provides unique insight into the body's insulin production
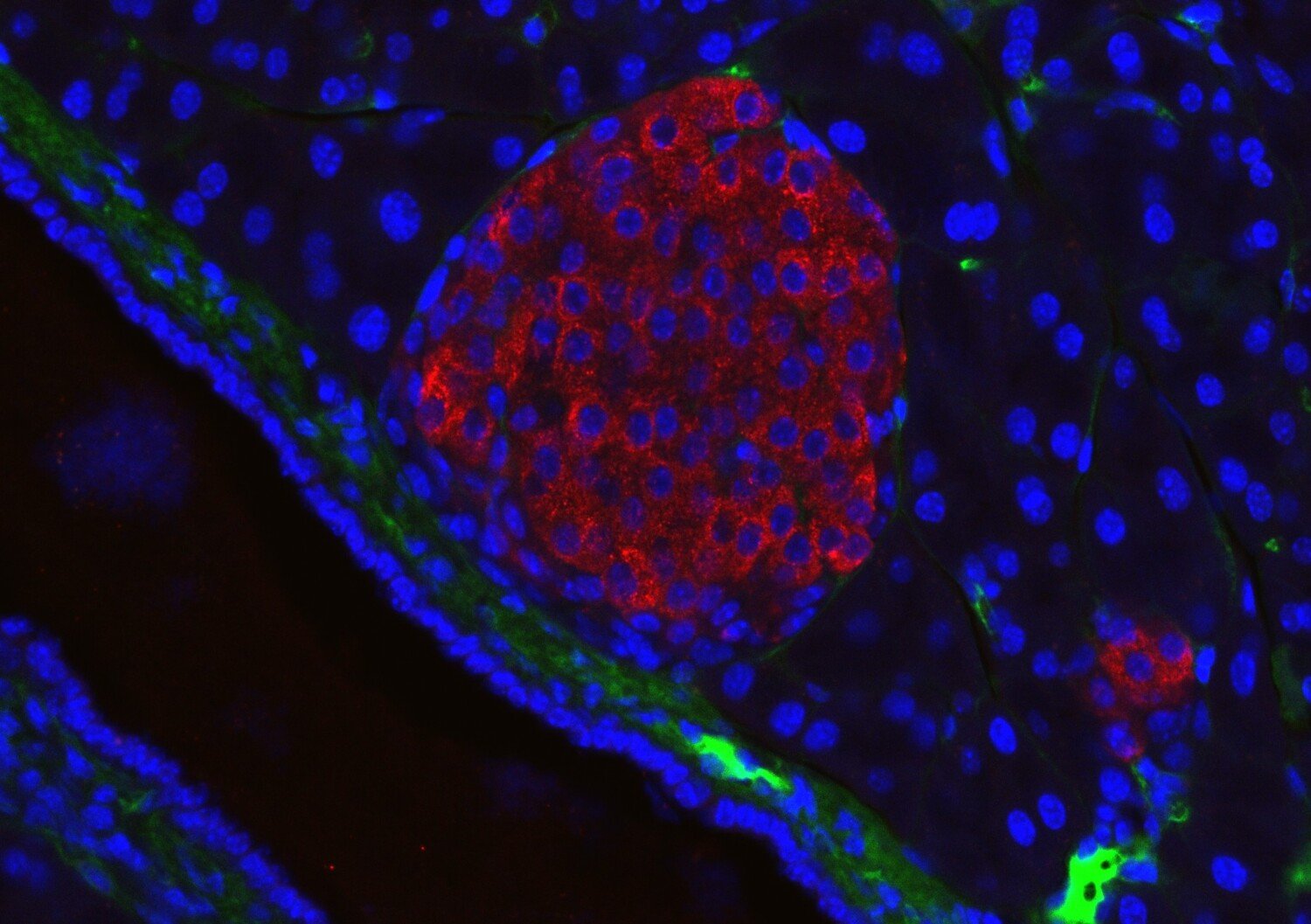
Researchers at Karolinska Institutet have developed a new method for studying the body's insulin-producing cells in mice. By placing small parts of the pancreas in an unexpected place in the body, the researchers can follow the work of the cells in detail. The study, which has been published in Nature Communications, may eventually contribute to better treatments for diabetes.
Understanding how the cells that control blood sugar work is crucial to preventing and treating diabetes. Researchers at Karolinska Institutet have now developed a technique that makes it possible to study these cells – the pancreatic islets of Langerhans – in living organisms with a high level of detail. The islets of Langerhans regulate blood sugar levels, and their dysfunction is central to the onset of type 2 diabetes.
Previously, researchers had to put animals to sleep to be able to study the cells, but anaesthesia affects both blood flow and cell activity. The new method provides a more realistic picture of how the cells work.
Cells' reaction to blood sugar
The researchers transplanted small islets of pancreatic tissue to the brain's outermost membrane, the dura mater, of mice. There, the tissue makes contact with blood vessels and nerves and can be studied through a small window in the skull bone. With advanced microscopy, the researchers were able to follow how the cells reacted to sugar in the blood and how their activity changed over time.

"The results show that the cells retain their ability to sense blood sugar and release insulin, even when they are in a completely new location. We were also able to see how anaesthesia affects the cells' signals, something that previously made many experiments difficult to interpret," says Dr. Philip Tröster, researcher at the Rolf Luft Research Centre for Diabetes and Endocrinology, Department of Molecular Medicine and Surgery, KI, and first author of the study.
To reduce stress and discomfort, the mice were given pain relief with buprenorphine after surgery and were cared for under sterile and controlled conditions. They were also gradually trained to get used to the special environment for microscopy and were able to move freely.
On the way to better treatments
Diabetes occurs when insulin production does not work properly. By understanding exactly how these cells work under normal conditions, researchers can develop better treatments and perhaps new ways to transplant cells into patients.

"Being able to study insulin-producing cells in waking animals gives us a unique opportunity to understand how they work in reality. It is an important step towards more accurate treatments for diabetes," says Professor Per-Olof Berggren at the Rolf Luft Research Centre for Diabetes and Endocrinology, Department of Molecular Medicine and Surgery, KI, who led the study.
In the long term, the method can be used to test how new drugs affect cell function or to improve transplants of insulin-producing cells to people with severe diabetes.
The study was funded by Karolinska Institutet, the European Research Council's (ERC) Advanced Grant, the Erling-Persson Family Foundation, the Jonas & Christina af Jochnick Foundation, the Novo Nordisk Foundation, the Swedish Diabetes Association, the Swedish Foundation for Strategic Research and the Swedish Research Council. Ethical approvals have been obtained in accordance with institutional and national guidelines for animal testing.
Publication
"Stable intracranial imaging of dura mater-engrafted pancreatic islet cells in awake mice", Philip Tröster, Montse Visa, Ismael Valladolid Acebes, Martin Köhler, Per-Olof Berggren. Nature Communications, online 18 November 2025, doi:10.1038/s41467-025-66057-4.
