Important redox systems of neglected tropical disease vectors characterised
Researchers from the Division of Biochemistry, in collaboration with scientists from University of L'Aquila in Italy, Rush University and University of California San Francisco in the US, have now for the first time characterized the crystal structure and activity of the B. malayi and O. volvulus antioxidant systems, thereby paving the way for the development of more efficient targeted redox-based therapies.
Two neglected tropical diseases, lymphatic filariasis and onchocerciasis (also known as “river blindness”) are transmitted by the vector parasites Brugia malayi and Onchocerca volvulus, respectively, leading to severe diseases and dehabilitation for millions of people worldwide. The current and often inefficient treatment strategies for these diseases focus on transmission prevention, which risk allowing for the development of drug resistance over time and are ill-suited to areas where coinfection with other parasites is prevalent. Researchers from the Division of Biochemistry, in collaboration with scientists from University of L'Aquila in Italy, Rush University and University of California San Francisco in the US, have now for the first time characterized the crystal structure and activity of the B. malayi and O. volvulus antioxidant systems, thereby paving the way for the development of more efficient targeted redox-based therapies. The results have been published in the journal Redox Biology.
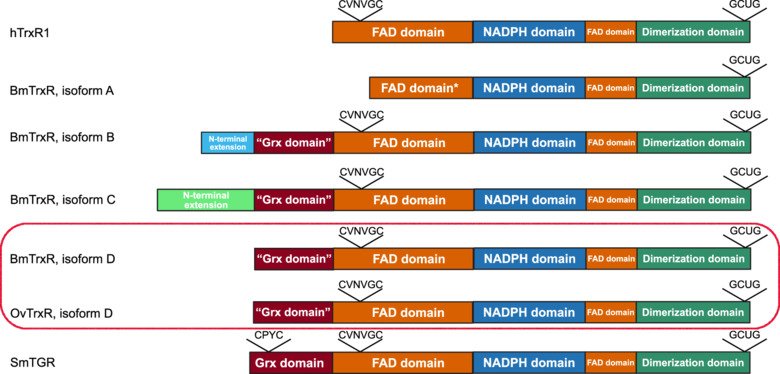
The researchers focused on investigating the thioredoxin systems of B. malayi and O. volvulus, knowing that these systems are important for detoxifying cells from oxygen radicals and for supporting a large number of reducing pathways that are required for maintaining cell and organism viability. The parasite thioredoxins (Trx) and selenium-containing thioredoxin reductases (TrxR) were discovered to carry highly unusual features with respect to their mammalian counterparts, but were nonetheless capable of direct interactions with some human antioxidant systems. These parasites’ TrxR were found to contain vestiges of the glutaredoxin (Grx) system capable of reducing glutathione (GSH), another important antioxidant, and have previously been regarded to be highly similar to the class of proteins known as thioredoxin glutathione reductases (TGR) present in the parasite Schistosoma mansoni. However, their activity profiles have now been found to more closely resemble those of mammalian TrxR than those of SchistosomaTGR. The functions of these new “Grx-like domains” remain to be discovered. At the same time, the activity of the filarial thioredoxin system with its counterpart in humans points to a previously unknown host-parasite interaction with implications for the broader effects of parasitic infections on host antioxidant systems.
The solved crystal structures of the B. malayi TrxR that the researchers were able to obtain have not only identified important and unique features of parasite thioredoxin reductases, but have also confirmed the mode of action and efficiency of human thioredoxin reductase inhibitors, such as auranofin and TRi-1. This could warrant the repurposing of TrxR inhibitors from chemotherapeutics to antiparasitic drugs. This research is the first step towards creating antiparasitic drugs capable of targeting both the transmission stage and ongoing disease, thereby bringing us closer to the eradication of lymphatic filariasis and onchocerciasis.
All crystal structures have been deposited in the protein database with IDs 7P0X, 7PUT and 7PVJ.
Publication: Fata F#, Gencheva R#, Cheng Q, Lullo R, Ardini M, Silvestri I, Gabriele F, Ippoliti R, Bulman CA, Sakanari JA, Williams DL&, Arnér ESJ&, Angelucci F&. Biochemical and structural characterizations of thioredoxin reductase selenoproteins of the parasitic filarial nematodes Brugia malayi and Onchocerca volvulus. Redox Biol. 2022 Mar 4;51:102278. doi: 10.1016/j.redox.2022.102278.
# shared first authors. & shared senior authors.
